Mytigate GmbH
Kontaktdaten
Adresse
Pharma Lane Risk Assessment
Improving Compliance and Reducing Transport Risk
INTRODUCTION TO MYTIGATE
Since the introduction of the EU GDP Guidelines in 2013, pharma companies and their forwarders are required to assure quality throughout their supply chain and to conduct a risk-based selection process for their transport partners. Until now, however, many have been unable to meet these requirements.
MYTIGATE - being an innovative, web-based service - offers a new solution that will help you to be compliant, and to select lanes using those supply chain partners that offer the highest quality and lowest risk.
Risks are continuously calculated, starting from the design phase of the lane through to the operating phase. The calculation of these risks is based on state-of-the-art, scientific-mathematical risk modelling using information from both supply chain partners (certificates, self-assessment of capabilities) and pharma companies (operational deviations, root cause data, temperature logger data).
By these means, MYTIGATE aims to be a must-have risk management standard within the pharma industry and to be the driver for innovation in the transport and supply chain industries.
The MYTIGATE risk management platform is a Software-as-a Service (SaaS) that was co-created with the pharma industry, working hand in hand with global forwarders to plan, monitor and improve the quality of pharma shipments in a legally compliant and safe manner.
KEY FEATURES
Product Specifications
Integrated product transport requirements
Reporting
Issue reports to regulators and document your risk-based lane assessment
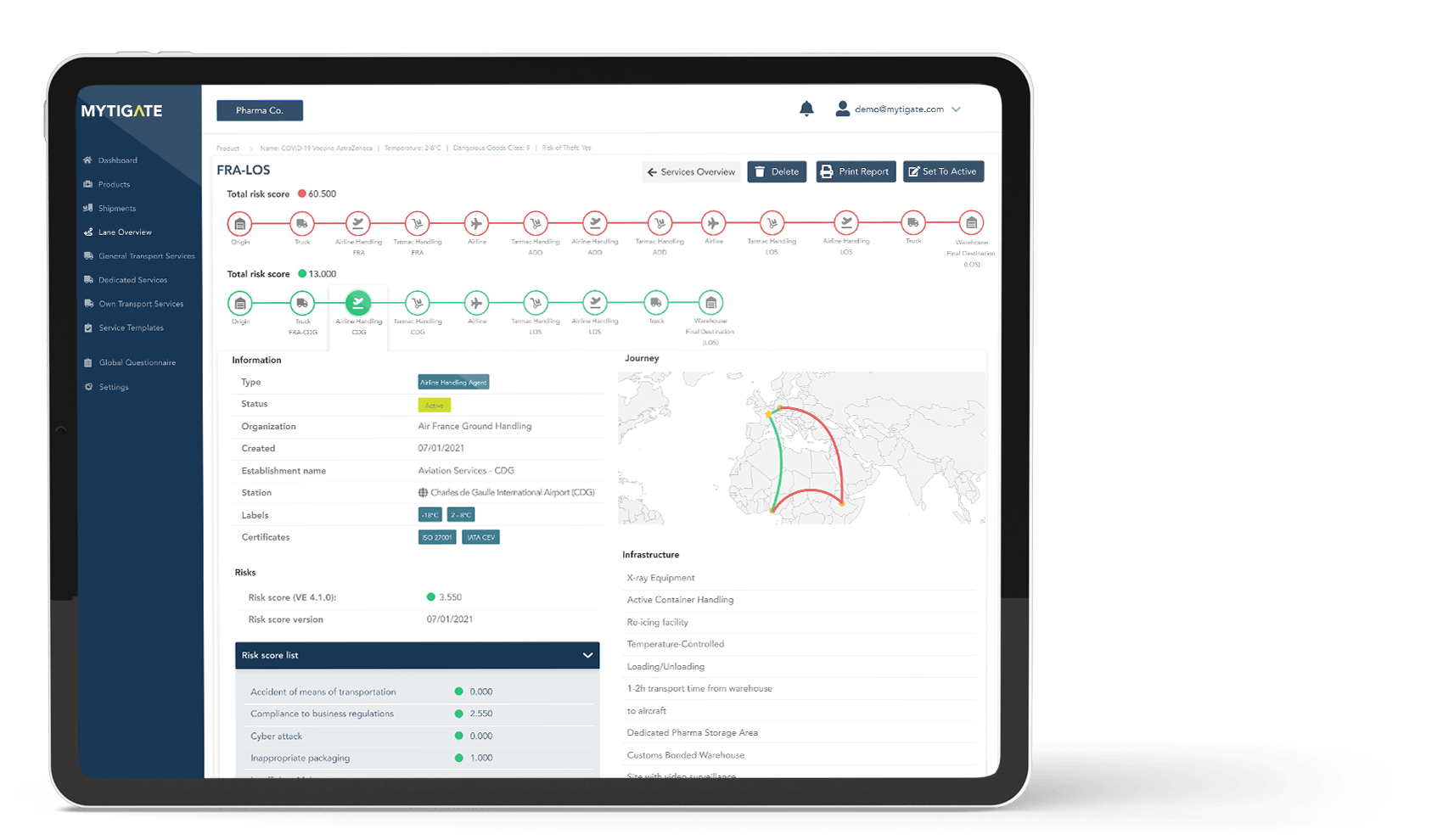
Lane Design
Digitise your lane management process from risk assessment to selection, qualification, and approval
Partner Profiles
Evaluate pharma-specific handling capabilities of major transport companies including airlines, handling agents, warehouses, and other logistics suppliers
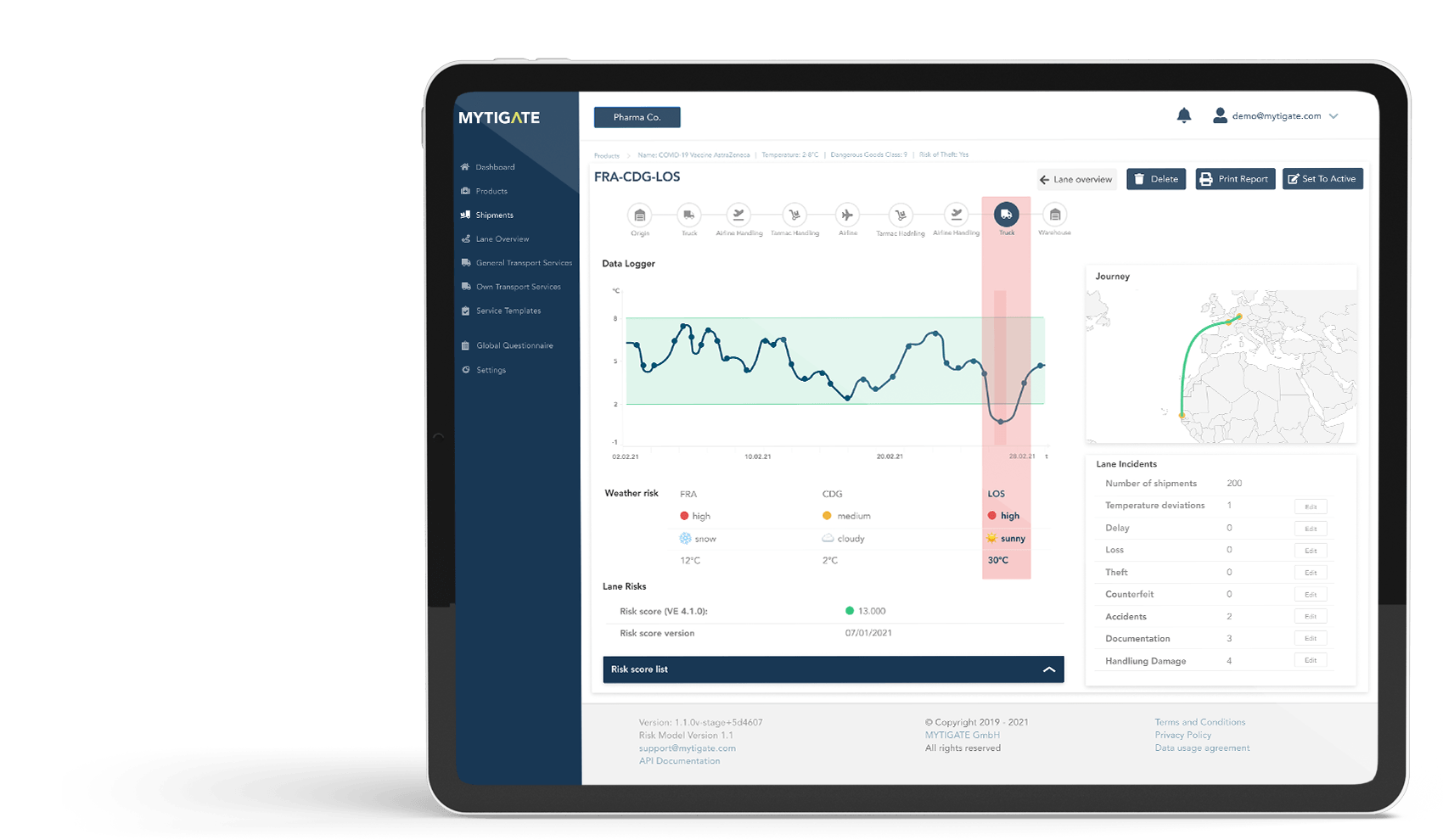
Temperature Data Logger
Continuously monitor the quality of transport on the selected routes by linking your own performance data (e.g. temperature logger data) to the shipments
External Risks
Integrated external weather risks and exposure risk
Lane Incidents
Realise savings in the areas of packaging, quality management, insurance, and product destruction
ABOUT US
MYTIGATE GmbH is based in the Rhine-/Main area, yet by embracing a fully digital work environment, our international team spans multiple countries including Canada, Germany, Russia, and Turkey. Our office is located in the House of Logistics and Mobility (HOLM) in Frankfurt, Germany. MYTIGATE commenced operations in April 2020 in order to commercialise the results of the research project „Pharma Supply Chain Risk Management“. This project started in 2017 and was funded by the LOEWE3 innovation fund of the state of Hesse in Germany and managed by Frankfurt University of Applied Sciences.
Working hand in hand with industry leaders and academic institutes, our vision is to become the quality and sustainability standard for both the pharma and the forwarder industry. To achieve this vision, we bring together the scientific collaborative efforts of the different parties involved, connecting academic and research knowledge with the industry.
Branche
SoftwareSoftware
Compliance ManagementQualitätsmanagement
Risk Management
Verkehrsträger
LandLuft
See